Cookie Notice
By continuing to use this website, you consent to our use of cookies and the other terms of our Privacy Policy.
For more information, please visit our Privacy Policy.
*In a post hoc analysis of CHAMPION PHOENIX, the absolute benefit of KENGREAL increased progressively with the number of high-risk lesion features treated and was greatest for patients with ≥3.2
Learn about clinical cases where KENGREAL may be appropriate.
In this 30-second video, see the effect of KENGREAL on the thrombotic response to laser-induced injury in the mouse cremaster muscle arteriole.3,4
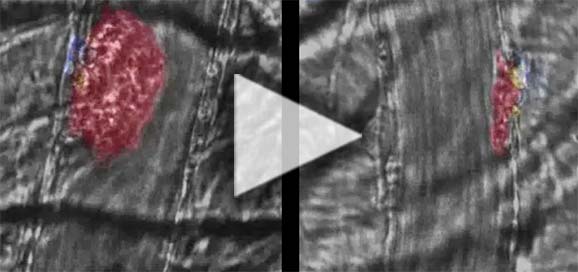
In this 30-second video, see the effect of KENGREAL on the thrombotic response to laser-induced injury in the mouse cremaster muscle arteriole.3,4
Information featured here includes in vivo data, the clinical significance of which has not been established.
In CHAMPION PHOENIX, KENGREAL provided significant risk reduction vs clopidogrel in the composite outcome of MACE (death, MI, IDR, and ST) at 48 hours, and the individual outcome of ST.1,5 Patients receiving KENGREAL had no statistically significant increase in GUSTO-defined severe bleeding or need for transfusion.5 Bleeding events of all severities were more common with KENGREAL than with clopidogrel.1
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
Open
Close